Cu to Cu2+ Oxidation or Reduction
Cu 2 I - Cu I 2 Step 2. The first visible signs of oxidation occurred in about 2 min.

Chapter 19 Electrochemistry Ppt Download
An increase in oxidation state is oxidation eg Zn and a decrease in oxidation state is reduction eg CuII.

. And identify what is being oxidized and what is being reduced. Which means Copper has lost 2 electrons. Cu Ag NO3- --- Ag Cu2 2NO3-Step 3.
Copper sulphate and Zinc b Calcium Carbonate and Magnesium c Iron sulphate and Zinc d Sodium chloride and. Cu sCu2 aqAg aqAg s Anode oxidation Cu s Cu2 aq 2e-Eo ox -Eo Cu -0337 v Cathode reduction Ag e- Ag s Eo red E o Ag 0800 v 2 x Ag e- Ag s 0800 v Cu s Cu2 aq 2e -0337 v Cu s 2Ag aq Cu2 aq 2 Ag s 0463 v Eo cell Or. This is an example of oxidation.
D reduction by LOSS of electrons 2. Assign oxidation numbers to each species. NHE 67 the higher CB.
B Cu2 will discharge easily at cathode. Oxidation means loss of electron and reduction means gain of electron. The present study reports on the in situ oxidation of copper to Cu 2 O and subsequent reduction to metallic copper in an environmental scanning electron microscope.
Als Cu 2 aq Al 3 aq Cus 1. OxidationReduction Net ionic equations Description. Cu 2 aq Cus reduction Als Al 3 aq oxidation 2.
A oxidation by GAIN of electrons 2. Comment Clark Bull-Misner 2 years ago Follow The FORMAL LOSS of electrons specifies oxidation and we introduce oxidation numbers as labels to make electron lossgain specific. Journal of Chemical Education Vol.
In both species of enzyme rapid reduction of Cu2 ions by substrate takes place. O a is Ag c is Cu and electrons flow from c to a O a is Ag c is Cu and electrons flow from a to c O a is Cu c is Ag and electrons flow. C oxidation by LOSS of electrons 2.
Cu - Cu2 2e-Loss of electron is Oxidation. Cu -2e Cu2 247 views Submission accepted by Praveen Upadhyay Eniola Kayode 10 mo Reduction 225 views Submission accepted by Praveen Upadhyay Starboy Ollie. 400 mL beaker 1 M Copper sulfate 1 M Sodium.
Is Cu to Cu2 oxidation or reduction. Algorithm for plotting titration curves Author. In this case Cu donates 2 electrons to form Cu2 ionthereforeits an oxidation process.
Electrochemical Cell Cu2 Zn2 Cu V oxidation ANODE e e reduction CATHODE - Zn Note that the reaction is reversed from previous slide ie Cu is oxidized. C Carbon tetrachloride is a non-electrolyte because it is a covalent compound. In this Zn is changing from 0 to 2 and this is an oxidation while CuII is changing to Cu and going from 2 to 0.
One of Redox rules states that increase in number of Oxidation connotes oxidation reaction and decrease in number of Oxidation connotes reduction reaction. Identify the process below as oxidation reduction or neither oxidation or reduction. A redox reaction is nothing but both oxidation and reduction reactions taking place simultaneously.
2Cu2 2 I- 2Cu I2 Electron loss is oxidation and electron gain is reduction. Please log in or register to add a comment. A The reaction takes place at anode.
The copper is reduced. To solve this you need oxidation number rules search the internet. Cu Ag NO3- --- Ag Cu2 2NO3-Cu is being oxidized and Ag is being reduced.
A Assign oxidation numbers for each atom in the equation. 69 No3 March 1992 p257 Letters. 56 Reduction of Cu2 by Aluminum Subjects.
Eo cell EoCathode - EoAnode right left reduction. OX Cu --- Cu2 RED Ag --- Ag. Cu2 2 E- Cu - Chemistry.
Separate the process into half reactions. Copper is OXIDIZED from copper metal mathCu 0 math to cupric ion mathCu 2 math ie. This galvanic cell uses the reaction.
Cu s Cu2 aq 2 e- Choose Ni2 aq 2 e- Ni s Choose Question 22 3 pts Which species functions as the oxidizing agent in the following reaction. The two half-reactions are as follows. The oxidation was carried out at approximately 125 μm oxygen pressure while the reduction was done at the same pressure using H 2 gas.
Again cuppric ion Cu2 gains electron and gets reduced to cuprous Cu ion and hence Cu2 ion is oxidizing agent. Some are more complex eg SO32- --- SO42-. Identify which reactants are being oxidized the oxidation number increases when it reacts and which are being reduced the oxidation number goes down.
It does not ionize and hence do not conduct electricity. State Whether the Following Conversion is Oxidation Or Reduction. It should be noted that although the CB energy of TiO 2 001 surface is capable to drive the reduction of Cu to Cu Cu Cu 052 V vs.
Click here to get an answer to your question Cu-Cu2 is an example of oxidation or reduction garimagaba334 garimagaba334 23112020 Chemistry Primary School answered Cu-Cu2 is an example of oxidation or reduction 2. Cu s 2 Ag aq 2 Ag s Cu2 aq. The conversion of Cu2 to Cu indicates.
Reduction CATHODE Zn Cu2Zn2 Cu 8 An electrolytic cell is an electrochemical cell in which an electric current drivesan otherwise nonspontaneous reaction. In the given reaction iodide losses electron and gets oxidized to iodine and hence iodide is reducing agent. In the active enzyme Cu ions are reactive to O2 but in the inactive enzyme Cu can be oxidized only by oxidizing agents such as H2O2 or ferricyanide or by a slow intermolecular electron transfer from Cu 615 to the active enzyme.
B reduction by GAIN of electrons 2. The oxidation state increases from zero to plus three 3 electrons lost. Cu 0 Cu 2 2.
The oxidation state decreases from 2 to zero 2 electrons gained. When a ball of aluminum foil is placed in a copper solution with chloride ions the copper ions are reduced to copper metal and a coating of copper is seen on the surface of the aluminum. Cu - Cu2 Means total oxidation number moved from 0 to 2.
F a C oxidation half cell reduction half cell Identify the composition of electrodes and the direction of electron flow.
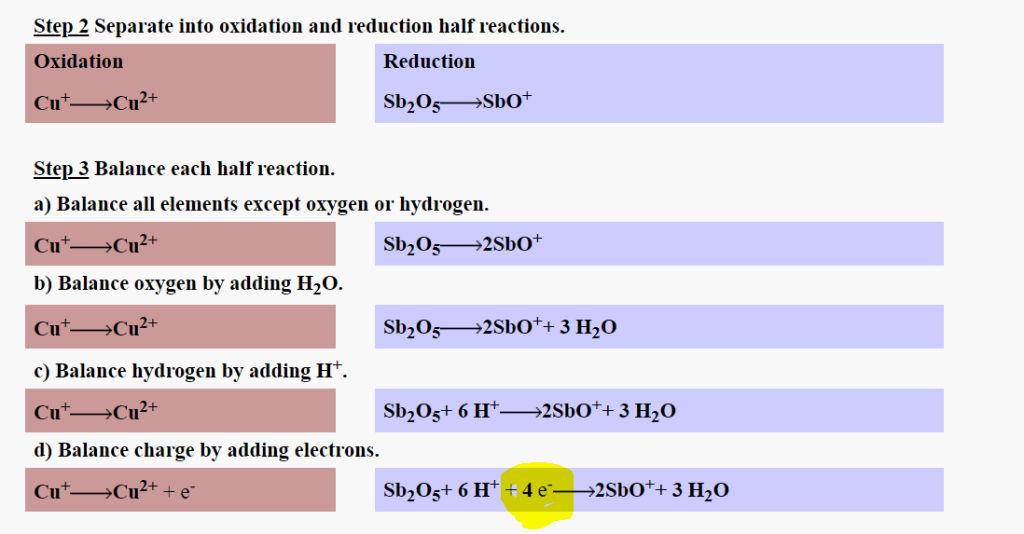
Solved Step 2 Separate Into Oxidation And Reduction Half Chegg Com

Chapter 17 Electrochemistry Ppt Download

Electrochemistry Experiment 12 Oxidation Reduction Reactions Consider The Reaction Of Copper Wire And Agno 3 Aq Agno 3 Aq Ag S Cu S Ppt Download

Comments
Post a Comment